
For medical device manufacturers, it's all about outcomes. With Propel, you can integrate quality beyond your QMS to ensure the best possible patient experience. Quality, compliance, and product all come together in a single platform to help your team improve lives.


Comply with regulations such as FDA and EU along with industry standards like ISO 13485, 21 CFR Part 11, and 21 CFR Part 820. Manage compliance with secure electronic signatures, password security, audit trails of each action, and risk management incorporated in all processes, along with a validation pack with executed IQ and OQ and template PQ scripts for all processes.



Incorporate Corrective And Preventative Action plans, implementation plans, risk analysis, and effectiveness checks. Get greater flexibility to configure the forms and workflows necessary to meet your customer's specific needs and expectations.



Easily notify and involve suppliers when a Supplier Corrective Action Request is in process, conducting supplier investigations and root-cause analysis in one information hub.



Integrate your Non-Conforming Material Report with other QMS processes such as CAPA and audits. Describe non-conforming material via configurable forms and workflows. Record non-conforming material and related supplier information to comply with FDA and ISO regulations, following industry best practices.



Resolve customer complaints and QA investigations to improve product safety and maximize customer loyalty. Fully integrate with Salesforce Service Cloud and other case management or customer service systems to leverage customer insights. Meet specific customer needs with fully configurable fields and workflows. Lower the risk of errors and non-compliance by incorporating decision trees that determine regulatory reporting requirements.



Manage the onboarding and qualification of new suppliers. Support audits and electronic approvals. Strengthen supplier collaboration to quickly resolve product and supply chain issues, minimize the risk of noncompliance, and improve product quality. Understand preferred and alternate sources, field replaceable units (FRU), cost parameters, and part availability.
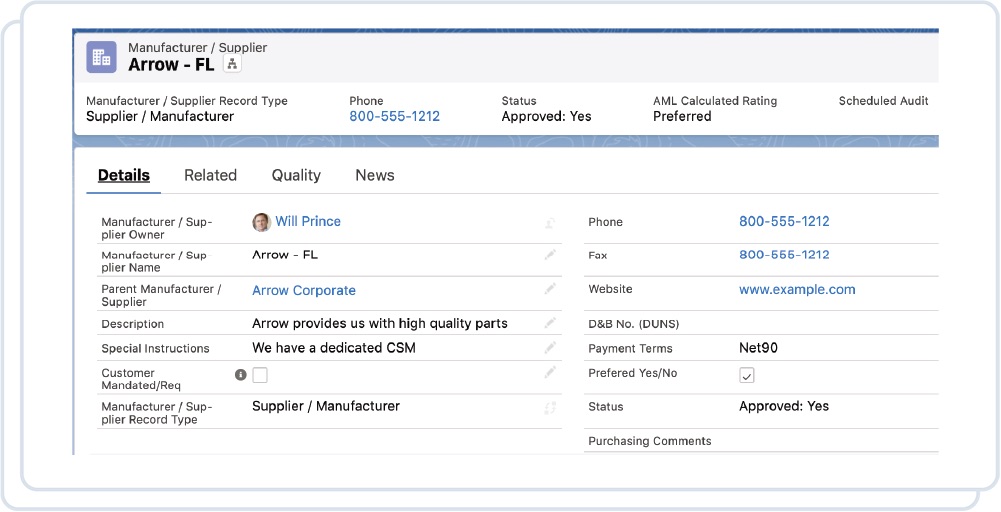


Quickly identify deviations from normal processes to ensure your organization is compliant with FDA and ISO regulations and practices.



Confidently pass audits and improve product and employee safety with a comprehensive training record management solution that includes full employee training transcripts, document-based training and quizzes, automated retraining, and training analytics. Streamline regulatory compliance by managing employee certification on the latest product changes and processes throughout the product lifecycle.



Incorporate risk management into all of your processes to minimize adverse reactions. Incorporate engineering change orders, CAPAs, and NCMRs into all of your processes. Precisely calculate the risk score for events based on parameters, such as issue severity and occurrence frequency, through a reportable risk management matrix.



Take preventive actions and make informed business decisions based on data-driven audit insights. Manage internal, external, and supplier audits and share information about upcoming audits and audit results with respective stakeholders.



Create Equipment and subsequent Maintenance records.
Determine and calculates if and when Equipment requires routine Calibration and/or Preventive Maintenance.
Manage Equipment Manufacturers, Site Location, Model and Serial Numbers, as well as Equipment Qualification and Software Validation.



Manage & track global product registrations and licensing for the countries in which you sell. Accurately plan, design & forecast product and sales into new markets. Streamline registration creation, maintenance, expiration and renewal. Ensure compliance, understand global impacts, and minimize inventory disruption.



Collaboratively manage the IFU throughout the entire product lifecycle. Publish accurate and localized versions of IFUs in the regions where the device is sold.
Eliminate errors typically associated with manual information entry and system integration.



Fully automate reporting through decision trees, regulated agencies tracking, support for electronic MedWatch FDA 3500A Form, and one-click submission. Keep track of the outbound electronic eSubmission package and all acknowledgements as proof for audits of reporting compliance.



Take Our Product Tour to See the Full Value








We’ve received your contact request. A member of our team will be reaching out shortly to set up a time to talk via email.
A link to our Product Tour has been sent to you via email.
The Buyer’s Kit you requested has been sent to your email. Share out with your team!