Learn how a collaborative, closed-loop system for managing product development and quality helps medical device manufacturers:
PLM isn't just for engineers anymore. Just like QMS isn't only focused on helping QA teams manage their own quality processes anymore. To navigate changing regulations and improve quality before shipping, you must integrate quality and compliance with product design. Taking a closed-loop approach makes it easier to stay compliant with changing regulations, design for quality, and deliver better patient outcomes.
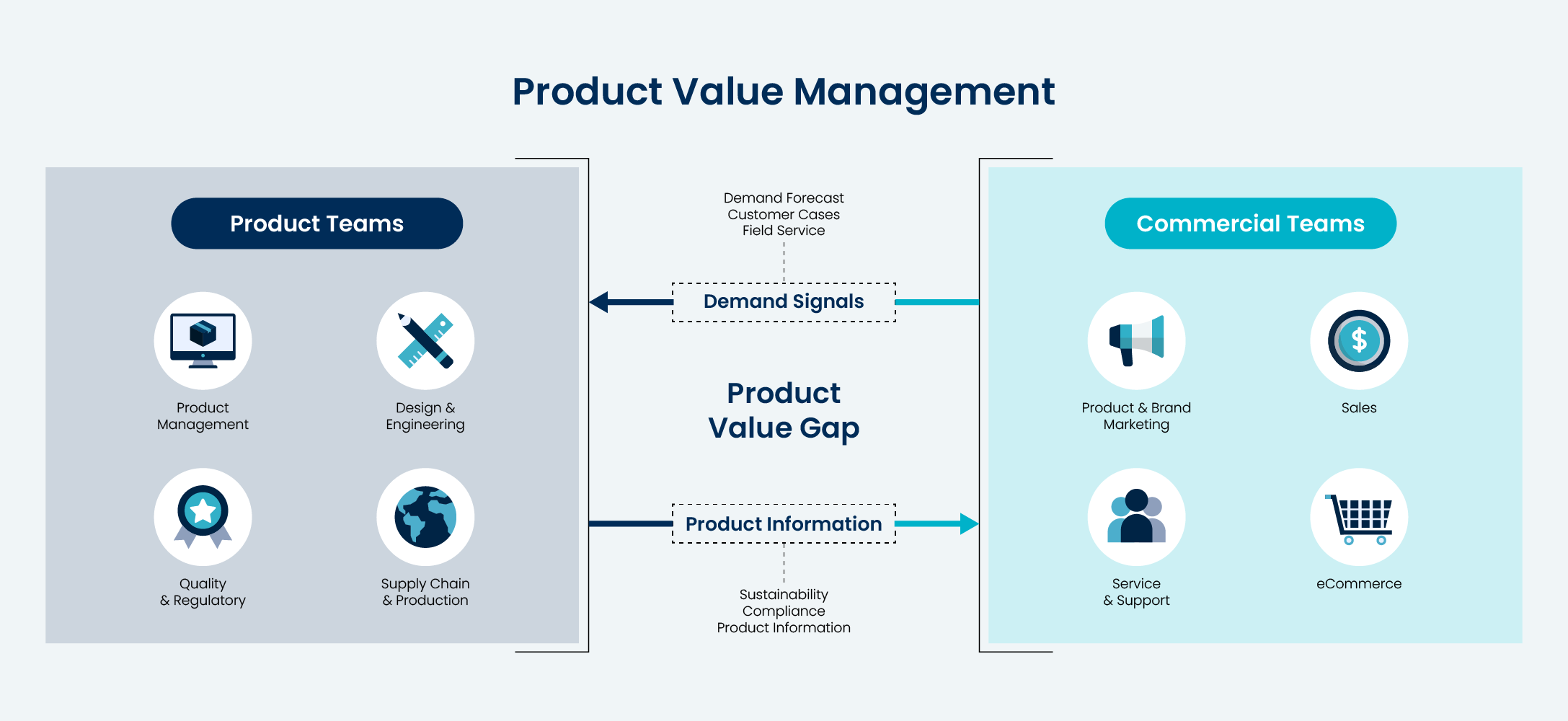
Learn about the clear signs of a product value gap, and what that means for IT leaders at product companies.


Insights and trends that will impact the future of manufacturing for years to come.



We’ve received your contact request. A member of our team will be reaching out shortly to set up a time to talk via email.